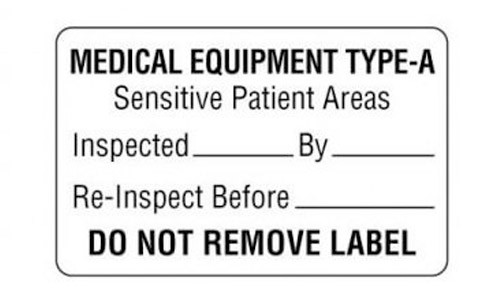
Medical Device Labels
New legislation affects over 4,100 medical device manufacturers in the U.S. and 21,000 worldwide. We can help ensure your labels meet new requirements for marking and durability. We offer materials that are UL certified and comply with UL/IEC 60601-1 3rd edition as amended. Medical device manufacturers come to ALI for labeling their equipment such as various imaging scanners, surgical instruments and other items used throughout the operating room. We understand the critical requirements that are needed for labels to withstand the conditions created through the sterilization process as well as wear and tear through continual use.
Contact ALI to discuss all of your medical device labeling needs.